Introduction
Various challenges and considerations exist when designing an efficient process for synthesising a target compound depending upon the scope of the project and industry.
SYNTHIA retrosynthesis software is a powerful tool for organic chemists that employs expert coded chemistry rules to predict feasible routes starting from commercially available raw materials. Using SYNTHIA to aid in the route design process allows a chemist to quickly select pathways that meet key criteria of the project, including using commercial starting materials within a set price range or avoiding certain hazardous reagents or reaction types.
In addition to the expert-coded rules, SYNTHIA utilises the published literature to incorporate previously reported synthetic steps alongside the computer-generated steps. Because the algorithms are designed to optimise potential routes based on price, step count, and atom economy, it often leads to more efficient and cost-effective routes than a chemist might design on their own or than those which have already been reported in literature.
To demonstrate the practical utility of incorporating SYNTHIA retrosynthesis software into the route planning process, this case study highlights the improved synthesis of a known compound that was designed with the help of SYNTHIA to reduce step count and increase overall yield. An evaluation regarding the Green Chemistry aspects of proposed routes was also performed using the DOZN tool.
Case study – lithium chromoionophore
Dyes have been used throughout human history to colour textiles. Early dyes consisted of natural products derived from insects, bark, leaves, berries, and fungi. The synthetic dye industry was born in 1856 when an 18-year-old William Henry Perkin serendipitously discovered mauveine during a failed attempt at synthesising quinine. Since the commercialisation of the first aniline dye, originally named ‘aniline purple’ for its rich purple hue, the dye industry has expanded beyond colouring textiles to include applications in photography and optical information recording media.
One such purple-blue coloured molecule with potential applications in optical information recording media is the indoaniline dye shown in Figure 1. This molecule also carries a mono aza-crown ether moiety, making it a chromoionophore which can selectively complex ions to cause a change in absorption and emission properties. Such ion-sensors have potential applications in trace metal detection in biological systems, as well as for molecular data processing.
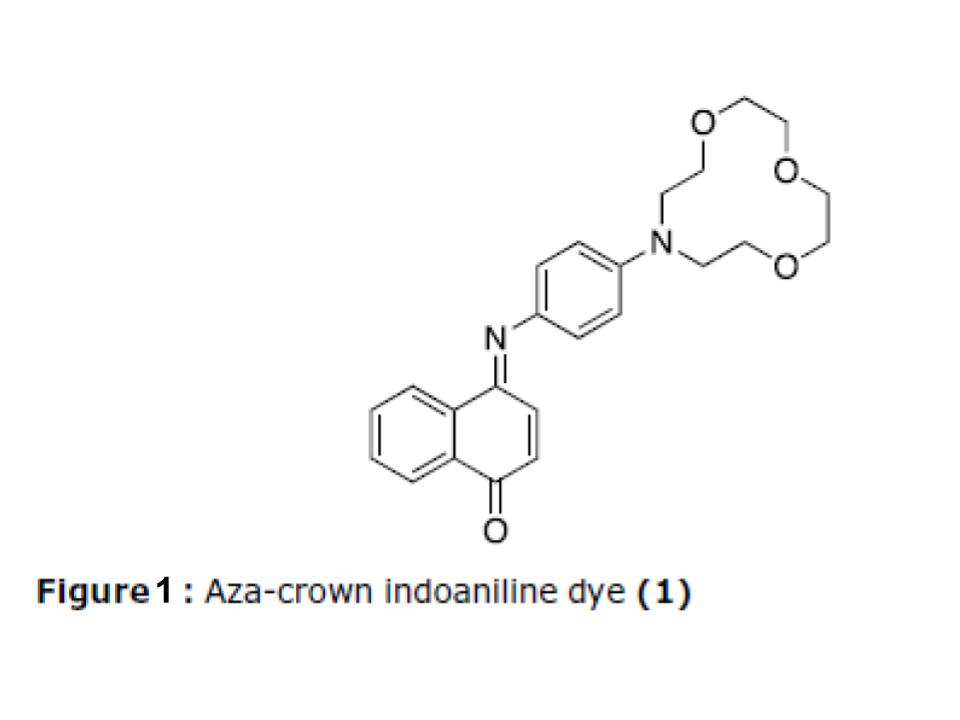
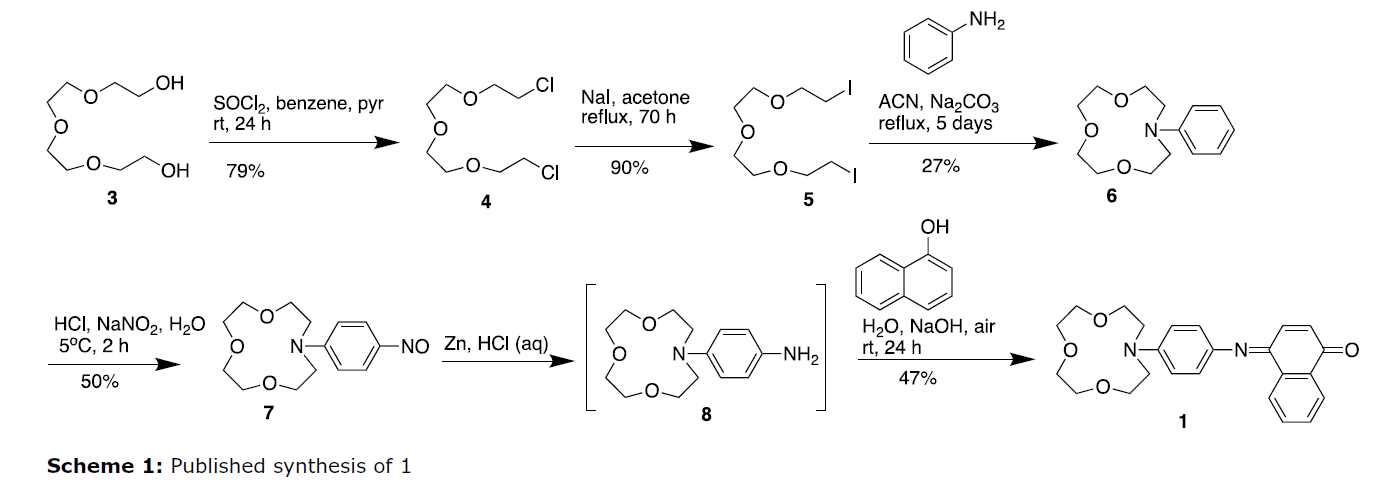
The synthesis of 1 was originally reported in 2000 by S.-H. Kim et al. (Scheme 1). The route starts with a series of functional group conversions from tetraethylene glycol 3, followed by a low-yielding intramolecular cyclization with aniline to generate the aza-crown ether 6. This is functionalized with HNO2 to generate the nitroso intermediate 7, which is subsequently reduced to amine 8 with Zn/HCl. The final step consists of a condensation with alpha-naphthol in basic solution at room temperature with air oxidation. This 6-step process produced 1 with a 5% overall yield.
When tasked with preparing this molecule, the goal was to find a shorter route to improve the overall yield and reduce the synthesis time. SYNTHIA identified a commercially available aza-crown ether that could be directly coupled with p-fluoronitrobenzene in the first step, eliminating the need for the first two functional group transformations and the low yielding cyclization. This reduced the overall cycle time for the steps to core structure 7 from almost nine days to one day, after which nitro intermediate 9 was isolated in 93% yield after purification via silica-gel chromatography.
In the second step, SYNTHIA recommended reduction of the nitro group, which was performed via catalytic hydrogenation with activated Pd on carbon. After filtration of the reaction mixture through celite and evaporation of the solvent, crude 8 was obtained as a pale yellow liquid and used immediately without further purification. Although not proposed by SYNTHIA, the last step was adapted from the original publication. The condensation of 8 with alpha-naphthol in the presence of hydrogen peroxide (H2O2) produced 1 in 20% yield as a purple-blue solid after purification via neutral alumina chromatography.
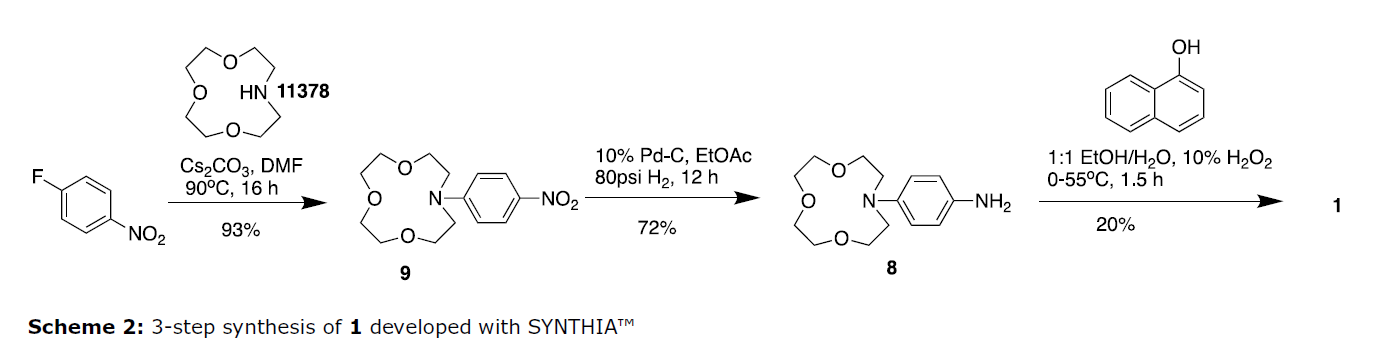
Using SYNTHIA to identify a key first step from commercially available starting materials allowed us to develop a 3-step synthesis to 1 with a 13% overall yield. This represented a 260% overall yield improvement and a 50% step reduction over the original synthesis. Additionally, the labour costs were reduced by 60%, which led to an overall savings of 49% compared to the original route, assuming a labour rate of $150/hr.
For more information visit www.synthiaonline.com
SYNTHIA™ Retrosynthesis Software is a registered trademark of Merck KGaA, Darmstadt, Germany
References
Perkin, W.H. The origin of the coal-tar colour industry, and the contributions of Hofmann and his pupils. J. Chem Soc. 1896, 69, 596-637. https://doi.org/10.1039/CT8966900596
Kim, S-H; Kim, J-W; Han, S-K; Koh, K-N; Park, S-W; Heo, N-H. Synthesis and X-ray structural characterization of indoaniline dye carrying a mono azacrown moiety. Dyes and Pigments, 2000, 46 (1), 43-48. https://doi.org/10.1016/S0143-7208(00)00027-9
Kim, S-H; Kim, J-W; Han, S-K; Koh, K-N; Park, S-W; Heo, N-H. Synthesis and X-ray
structural characterization of indoaniline dye carrying a monoazacrown moiety. Dyes
and Pigments, 2000, 46 (1), 43-48. https://doi.org/10.1016/S0143-7208(00)00027-9
Goosseens, J-F; Thuru, X.; Bailly, C. Free Rad. Properties and reactivity of the folic
acid and folate photoproduct 6-formylpterin. Biol. And Med. 2021, 171, 1-10. https://doi.org/10.1016/j.freeradbiomed.2021.05.002
Thijssen, H.H.W. A simple method for preparing 2-amino-4-hydroxy-6-formylpteridine,
a precursor of the pteridine substrate of dihydropteroate biosynthesis. Analytical
Biochemistry, 1994, 54, 609-611. https://doi.org/10.1016/0003-2697(73)90394-1
Freisleben, A.; Schieberle, P.; Rychlik, M. J. Syntheses of Labeled Vitamers of Folic Acid
to Be Used as Internal Standards in Stable Isotope Dilution Assays. Agric. Food Chem.
2002, 50, 4760-4768. https://doi.org/10.1021/jf025571k
Zav'yalov, S.I., Zavozin, A.G. & Ezhova, G.I. Regioselective syntheses of substituted
pteridines. Russ. Chem. Bull. 1981, 30, 2183–2185. https://doi.org/10.1007/BF01094658
Waring, P.; Armarego, W. L. F. Pterins. X. A New Preparation of 6-Hydroxymethylpterin
from 6-Methylpterin. Aust. J. Chem. 1985, 38, 629-631. https://doi.org/10.1071/CH9850629